Granules for Polyethylene bottles are imported from renowned manufacturers in Europe.
Globally preferred single-step closed form-fill-seal technology is employed for manufacturing Large Volume Parenterals (LVP). Each bottle is formed, filled and sealed in a controlled environment without human intervention.
This technology uses effective zeta potential charged filtration technique; solution and air are filtered several times through different size micron filters.
Our product range in LVPs comprises of common solutions, electrolyte replacements & diuretics in pack size ranging from 100 ml to 1000 ml.
Product Range
- » 5% Dextrose IV
- » 10% Dextrose IV
- » 25% Dextrose IV
- » 5% Dextrose & NS I.V.
- » 0.9% Sodium Chloride IV
- » Ringer Lactate IV
- » Mannitol
- » 5% Dextrose and Electrolyte – M
- » 5% Dextrose and Electrolyte – P
- » 5% Dextrose and Electrolyte – G
- » 5% Dextrose and Electrolyte – E
- » 5% Dextrose and 0.22% NaCI
- » 5% Dextrose and 0.33% NaCI
- » 5% Dextrose and 0.4
- » 5% NaCI
- » 10% Invert Sugar IV
- » Invert Sugar IV
- » Levofloxacin
- » Ofloxacin Infusion
- » Ciprofloxacin
- » Metronidazole
- » 0.2% Metronidazole & 5% Dextrose
- » Linezolid
- » Sodium Chloride Injection

Water undergoes several purification processes such as softening, reverse osmosis, demineralization & distillation before it reaches filling station. In-process tests are carried out by in-house laboratory at each stage according to GMP norms.
LDPE granules are imported from France & Germany, which undergo several physical, chemical & biological tests by in-house laboratory and other independent Government approved laboratories before it is approved for use in production.
Water for injections is manufactured through single stage form-fill-seal technology of Switzerland. The entire manufacturing process is fully automatic & does not require human intervention; the entire process is under horizontal laminar air flow. Further the filled ampoules are subject to terminal sterilization which leaves no room for contamination.
The state-of-the-art manufacturing plant is backed by FDCA approved technical staff, all the members of which have experience of more than 10 years in SWFI manufacturing industry.
The product is WHO – GMP certified, it is registered and exported to many countries around the world due to its quality and efficacy.
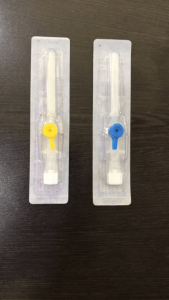
Imported P.T.F.E. Catheter which is best bio compatible material with least coefficients of friction .It is ultra thin wall and tapered by Imported tipping dies for easy penetration in vein. The P.T.F.E. catheter, designed and made with double tapered formed tip and siliconised, offers lower resistance during insertion resulting in less trauma to the patient, avoiding fold back. With Trisectional bevel (Tribevel) needles
- Medical grade paper for primary packaging complying with standard ISO 868.
- Labelling standard – ISO15223:2007 and graphical
- symbol as per standard EN 980:2008.
- Virgin plastic manufactured by reputed companies.
- Sampling standard as per ISO 2859:1999.
- Individually sterile & blister packed.

“Ribbon” Packaged, Single use syringes with needles mounted on top are synonymous with quality.
Product Specification:
- » Luer mount Single use syringes with & without Needle.
- » Manufactured as per IS:10258/ ISO:7886-1
Components:
Barrels are made of nontoxic, medical-grade polypropylene compatible with any medication.
The tip of the barrel is of two types – luer mount and luer lock. Both have a 6% luer taper as per ISO:594.
Plungers are made of nontoxic, medical-grade polypropylene compatible with any medication.
Gaskets are made of natural rubber which is chemically inert and compatible with medication for short term contact drug delivery application.
Packing:
Single use syringes are individually RIBBON PACKED in a double-laminated plastic film.